Lead-Acid Battery Structure and Working Theory: Complete Guide
Understanding how lead-acid batteries work is essential for anyone involved in battery maintenance, repair, or selection. Whether you’re a mechanic, engineer, or DIY enthusiast, grasping the fundamental principles behind these electrochemical powerhouses will help you make better decisions about battery care and troubleshooting. This comprehensive guide explores the intricate structure and working theory of lead-acid batteries, providing the knowledge foundation you need to work with these systems effectively.

The Fundamental Chemistry of Lead-Acid Batteries
Lead-acid batteries operate on a reversible electrochemical reaction that converts chemical energy into electrical energy and vice versa. The basic reaction involves lead dioxide (PbO₂), sponge lead (Pb), and sulfuric acid (H₂SO₄) dissolved in water.
Primary Chemical Reactions
Discharge Reaction:
- Positive plate: PbO₂ + HSO₄⁻ + 3H⁺ + 2e⁻ → PbSO₄ + 2H₂O
- Negative plate: Pb + HSO₄⁻ → PbSO₄ + H⁺ + 2e⁻
- Overall: PbO₂ + Pb + 2H₂SO₄ → 2PbSO₄ + 2H₂O
Charge Reaction: The process reverses during charging, converting lead sulfate back to active materials.
This fundamental chemistry explains why lead-acid batteries are rechargeable and why certain problems like sulfation occur when batteries are left discharged for extended periods.
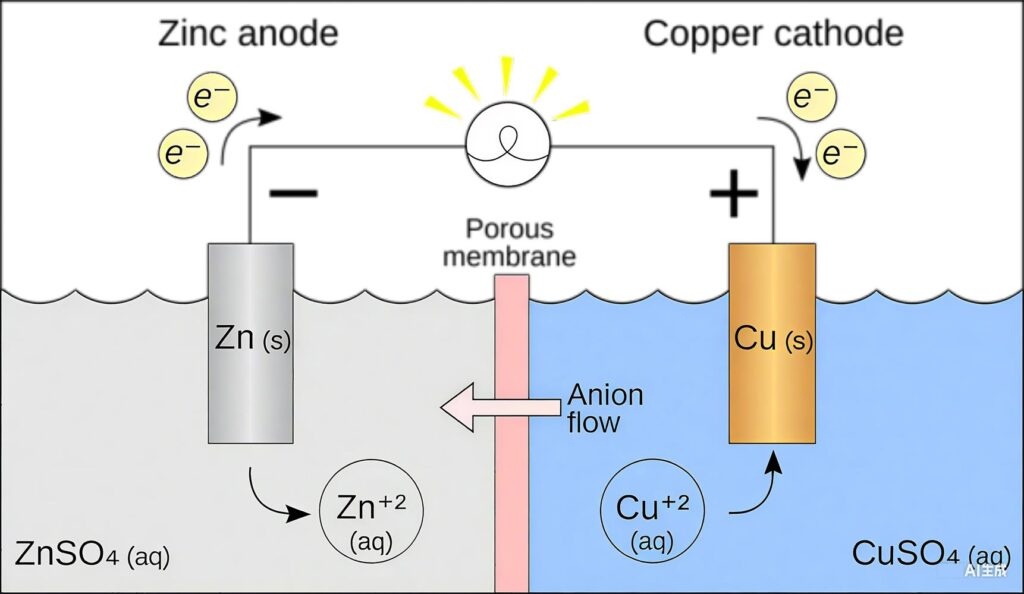
Core Components and Structure
1. Battery Plates: The Heart of Power Generation
Positive Plates (Cathode)
- Made of lead dioxide (PbO₂) pressed into a lead-antimony or lead-calcium grid
- Brown/chocolate color when healthy
- Generates positive current during discharge
- Grid provides mechanical support and electrical conductivity
Negative Plates (Anode)
- Composed of sponge lead (Pb) in a similar grid structure
- Gray color with porous, spongy texture
- Accepts electrons during discharge
- Larger surface area maximizes reaction sites
Grid Technology
- Lead-Antimony Grids: Traditional design, better deep-cycle performance
- Lead-Calcium Grids: Lower water loss, reduced self-discharge
- Lead-Tin Grids: Enhanced corrosion resistance
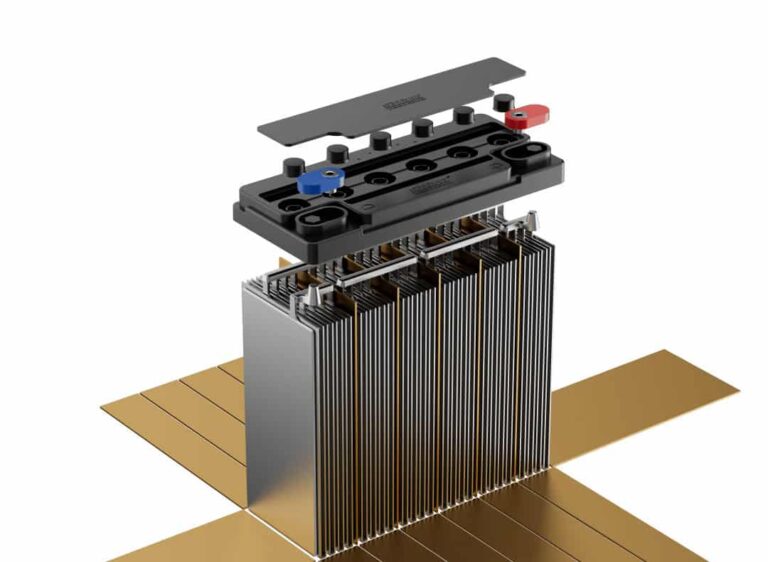
2. Separators: Preventing Short Circuits
Separators are crucial components that prevent physical contact between positive and negative plates while allowing ion flow:
Materials Used:
- Microporous rubber: Traditional, reliable separator material
- Fiberglass: Better acid resistance and ion flow
- Polyethylene: Lightweight with excellent chemical resistance
Functions:
- Prevent internal short circuits
- Allow electrolyte circulation
- Provide mechanical support
- Resist acid degradation
3. Electrolyte: The Conductive Medium
The electrolyte is a solution of sulfuric acid (H₂SO₄) and distilled water:
Concentration Levels:
- Fully charged: 1.265-1.280 specific gravity (35-38% acid)
- Discharged: 1.120-1.150 specific gravity (20-25% acid)
- Frozen electrolyte: Below 1.200 specific gravity at 0°F
Functions:
- Provides ions for electrochemical reactions
- Carries current between plates
- Participates directly in charge/discharge chemistry
- Affects battery capacity and performance
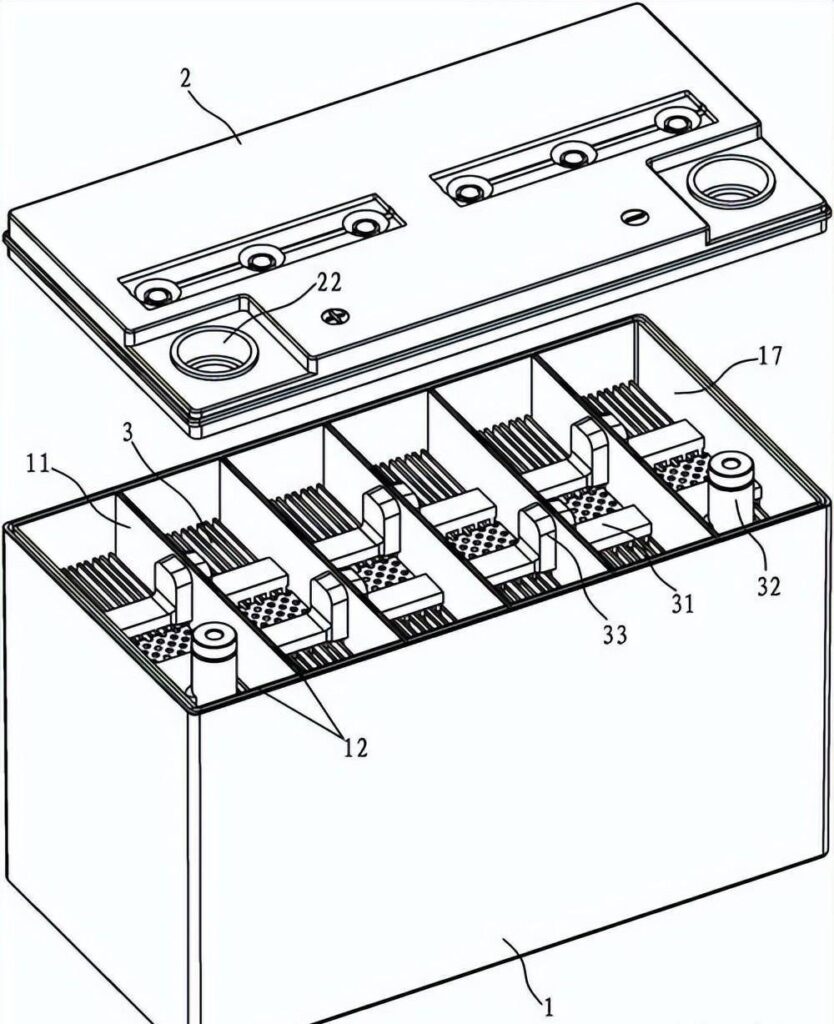
4. Battery Case and Cover
Case Materials:
- Polypropylene: Most common, excellent chemical resistance
- ABS plastic: Higher impact resistance
- Hard rubber: Traditional material, still used in some applications
Design Features:
- Individual cell compartments prevent electrolyte mixing
- Sediment wells catch active material that falls from plates
- Lifting handles for safe transportation
- Venting systems for gas release
Design Features:
- Individual cell compartments prevent electrolyte mixing
- Sediment wells catch active material that falls from plates
- Lifting handles for safe transportation
- Venting systems for gas release
Battery Cell Configuration
Single Cell Structure
Each cell produces approximately 2.1 volts when fully charged:
- Nominal voltage: 2.0V per cell
- Fully charged: 2.1-2.15V per cell
- Discharged: 1.8V per cell (cutoff voltage)
Multi-Cell Batteries
12V Battery: 6 cells in series (6 × 2.1V = 12.6V) 6V Battery: 3 cells in series (3 × 2.1V = 6.3V) 2V Battery: Single cell (used in large stationary installations)

Types of Lead-Acid Batteries
1. Flooded (Wet Cell) Batteries
Structure:
- Plates suspended in liquid electrolyte
- Removable caps for maintenance
- Vented design allows gas escape
Advantages:
- Lowest cost per amp-hour
- Proven technology
- Easily serviceable
Disadvantages:
- Requires regular maintenance
- Can spill electrolyte
- Generates hydrogen gas

2. Absorbed Glass Mat (AGM) Batteries
Structure:
- Electrolyte absorbed in glass mat separators
- Tightly compressed plate assembly
- Sealed, valve-regulated design
Advantages:
- Maintenance-free operation
- Spill-proof design
- Lower self-discharge rate
- Better vibration resistance
Disadvantages:
- Higher cost
- Sensitive to overcharging
- Cannot be opened for service

3. Gel Cell Batteries
Structure:
- Electrolyte immobilized in silica gel
- Sealed construction
- Pressure relief valves
Advantages:
- Deep discharge tolerance
- Excellent temperature performance
- No electrolyte stratification
Disadvantages:
- Most expensive option
- Sensitive to charging voltage
- Lower power density

Electrochemical Processes During Operation
Discharge Process
- Current Flow: Electrons flow from negative to positive terminal through external circuit
- Ion Movement: Sulfate ions (SO₄²⁻) move to both plates
- Chemical Conversion: Both plates convert to lead sulfate (PbSO₄)
- Water Formation: Water is produced at positive plate
- Acid Consumption: Sulfuric acid is consumed, reducing specific gravity
Charge Process
- External Power: Charger forces current in reverse direction
- Chemical Reversal: Lead sulfate converts back to active materials
- Acid Regeneration: Sulfuric acid concentration increases
- Gas Evolution: Hydrogen and oxygen gases produced near full charge
- Equalization: Overcharging helps balance cell voltages
Lead acid battery Maintenance and Monitoring
Ready to dive deeper into battery maintenance? Understanding these fundamental principles provides the foundation for effective lead-acid battery repair and reconditioning. With this knowledge, you can better diagnose problems, select appropriate batteries, and implement proper maintenance procedures.
Conclusion
The lead-acid battery remains one of the most reliable and cost-effective energy storage solutions available today. Understanding its structure and working theory is essential for anyone who works with these systems, whether for automotive, marine, backup power, or industrial applications.
The key to successful battery management lies in understanding the delicate balance of chemical reactions, physical structures, and operating conditions that determine battery performance and lifespan. By grasping these fundamental principles, you can make informed decisions about battery selection, maintenance, and troubleshooting that will maximize your investment and ensure reliable power when you need it most.
This knowledge also forms the foundation for advanced battery management techniques and helps you understand why certain maintenance procedures are necessary. Whether you’re maintaining a single automotive battery or managing a large battery bank, these principles remain constant and essential for optimal performance.




